In addition to induction therapy to achieve both histologic remission and clinical improvement/remission, effective management of EoE also requires long term maintenance treatment. This is to reduce the risk of both histological and clinical relapse and the potential for remodelling, including the development of strictures (small calibre oesophagus), resulting in the worsening of symptoms which could be refractory to drug therapy.1 Jorveza® represents the first and, to date, only approved pharmacological treatment option for EoE. Its label includes both induction and maintenance therapy in adults with EoE.2
Jorveza® contains the proven drug budesonide (a topical corticosteroid), which has been used to treat disorders such as inflammatory bowel disease (including Crohn’s disease and ulcerative colitis), asthma and chronic obstructive pulmonary disease (COPD) for many years. Importantly, budesonide has low systemic absorption, reducing the risk of systemic adverse events.3
Jorveza® was developed specifically for the treatment of EoE to achieve high therapeutic levels of budesonide at the site of inflammation in the oesophagus of a patient with EoE. Dissolution of the budesonide from the Jorveza® tablet is initiated when the patient places it on their tongue and gently presses it against the bridge of their mouth, which is usually completed over 2 to 5 minutes (but can take up to 10 minutes or longer in some patients). The slight effervescence in the formulation induces salivation, with the dissolved budesonide mixing with this saliva, making it a biologic vehicle for the corticosteroid. The patient is instructed to swallow the saliva ‘little-by-little’ as it is produced. The swallowed saliva coats the epithelial layer of oesophagus and due to its mucoadhesive properties, it may extend the residence time of the budesonide, thereby potentially increasing its uptake into the oesophageal mucosa (see Fig. 1).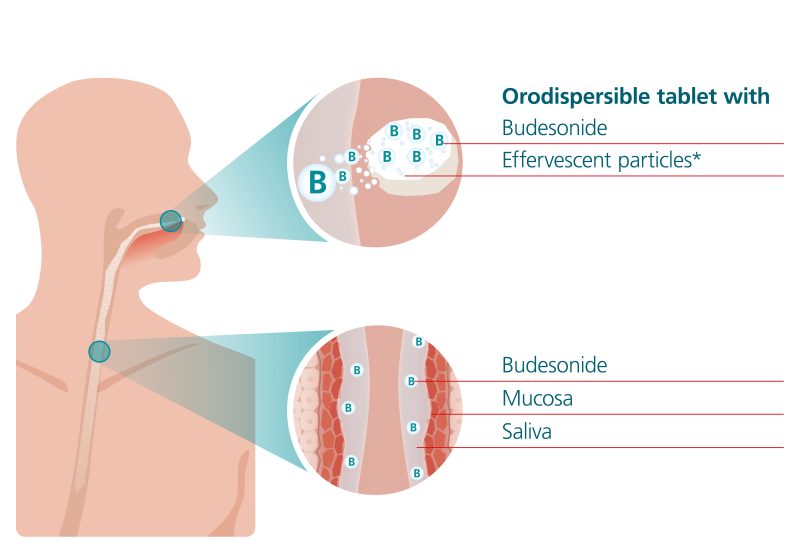
PLEASE REVIEW PRODUCT INFORMATION BEFORE PRESCRIBING.
COPIES OF THE PRODUCT INFORMATION FOR JORVEZA® CAN BE REQUESTED BY CALLING DR FALK PHARMA AUSTRALIA PTY LTD ON 1800 DRFALK (1800 373 233), OR AT THERAPEUTIC GOODS ADMINISTRATION.
|
PBS Information: Authority required for the treatment of EoE in adults. |