1. How effective is Jorveza® for induction of remission?
In the randomised, double-blind, placebo-controlled, Phase III (registration) study for Jorveza® in adult patients with active EoE, 57.6% of patients receiving the 1 mg BID regimen were in clinicohistologic remission at week 6 (primary endpoint) vs. 0% in the placebo group (p<0.00001). When Jorveza® treatment was extended to a total of 12 weeks, the clinicohistologic remission rate increased to 84.7% (Fig. 1). Furthermore, 93.7% of patients in the Jorveza® group were in histologic remission at week 6 (vs. 0% of the placebo group; p<0.0001)1, highlighting the importance of achieving histologic remission first to allow oesophageal healing before symptom improvement can occur.
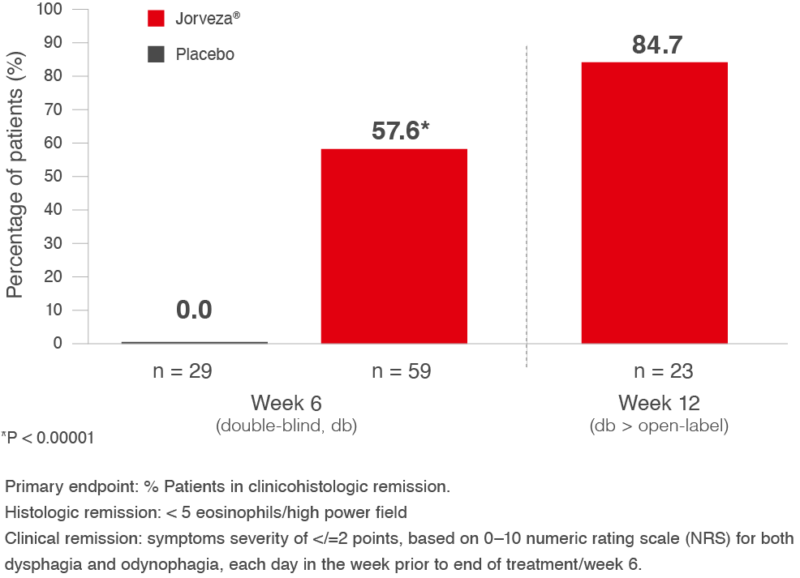
Fig. 1: % Patients in clinicohistologic remission at week 6 or week 12 following Jorveza® 1 mg BID treatment1
Furthermore, a complete normalisation of oesophageal appearance was documented in 61% of patients receiving Jorveza® vs 0% in placebo group; p<0.0001)1, based on a treatment-blinded assessment using the Endoscopic Reference Score (EREFs). [The EREFs is used to determine severity of key endoscopic findings: oedema, rings, exudates (markers of inflammation) and furrows, strictures and crepe-paper oesophagus (markers of fibrosis). Each of these findings is graded from normal/none to severe/present/absent, related to the specific parameter].
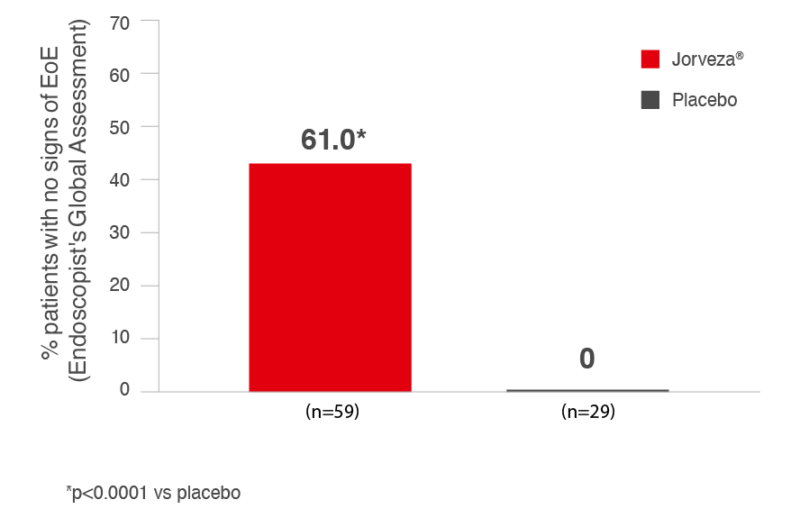
Fig. 2: Percentage of Patients in Endoscopic Remission with Jorveza Treatment (1 mg BID) at week 6.1
2. How effective is Jorveza® for maintenance therapy?
As a chronic inflammatory disease, the risk of histologic and thus clinical relapse in EoE patients is high following successful induction treatment if long-term and effective maintenance therapy is not initiated. To evaluate the efficacy and safety of long-term maintenance therapy with Jorveza® (1 mg BID) in adult EoE patients in clinicohistologic remission following prior placebo-controlled induction treatment with Jorveza®, patients were enrolled in a prospective, randomised (1:1:1), double-blind study to receive either Jorveza® 0.5 mg BID or Jorveza® 1 mg BID, or matching placebo and were followed for 48 weeks.2 The primary endpoint was the percentage of patients without treatment failure (i.e. maintaining clinicohistologic remission), defined fulfilling ALL of the following criteria as: absence of clinical relapse; absence of histological relapse; absence of food impaction requiring endoscopic intervention; no need of dilation and/or no premature withdrawal for any reason. At week 48, 73.5% and 75.0% of the patients in the Jorveza® 0.5 mg or Jorveza® 1 mg BID treatment groups, respectively, were still in clinicohistologic remission vs. 4.4% with placebo (p<0.001; see Fig. 3).3
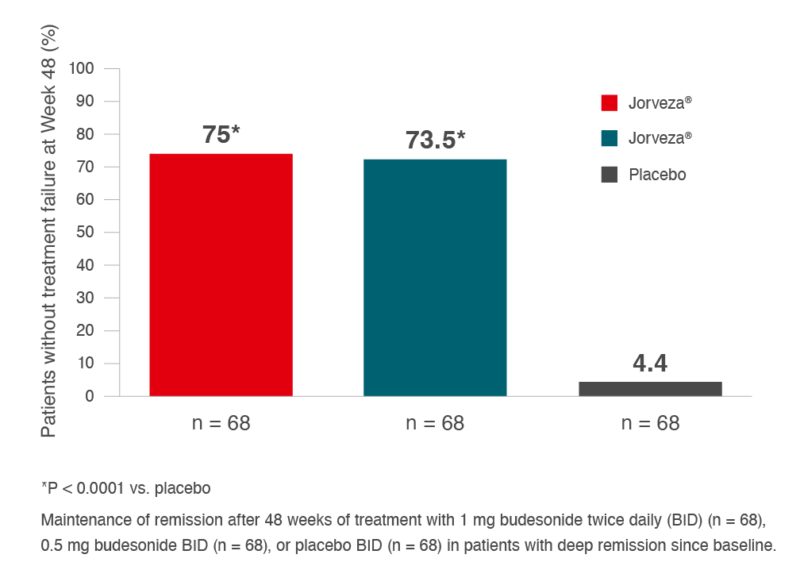
Fig. 3: Maintenance of clinicohistologic remission over 48 weeks in adult patients with EoE with
Jorveza® treatment, following prior induction of clinicohistologic remission with Jorveza®.3
In addition, in the placebo group, 89.7% of subjects experienced histological relapse (≥48 eos/mm2 hpf; ≥15 eos/hpf) versus 10.3% of subjects treated with 1 mg Jorveza® BID and 13.2% of subjects treated with 0.5 mg Jorveza® BID regimens.3
Based on the documented rate of clinical relapse, the time to clinical relapse was also estimated for the 3 treatment groups (Fig. 4). For the placebo group, the median time to relapse was 87 days, while for the two Jorveza® treatment groups, this was >350 days.3
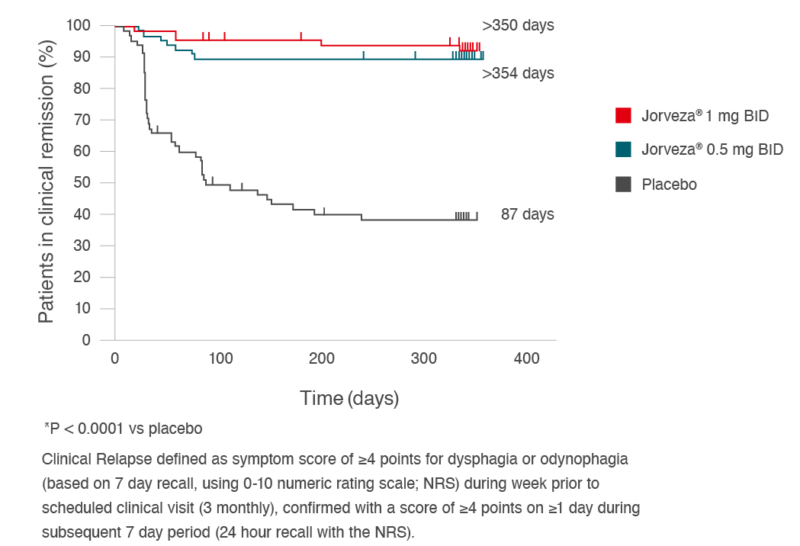
Fig. 4: Time to clinical relapse for two Jorveza® regimens (0.5 mg BID or 1 mg BID) vs. placebo in the 48-week maintenance study.3
Recommended Dosage for Induction & Maintenance Treatment for Jorveza®
The recommended daily dose for induction treatment is 2 mg Jorveza® as one 1 mg tablet in the morning and one 1 mg tablet in the evening. The usual duration of induction treatment is 6 weeks. For patients who are not appropriately responding during the 6 weeks, treatment can be extended up to 12 weeks.4
The recommended daily dose for maintenance of remission is 1 mg Jorveza® as one 0.5 mg tablet in the morning and one 0.5 mg tablet in the evening, or 2 mg Jorveza® as one 1 mg tablet morning and one 1 mg tablet in the evening, depending on the individual clinical requirement of the patient.4
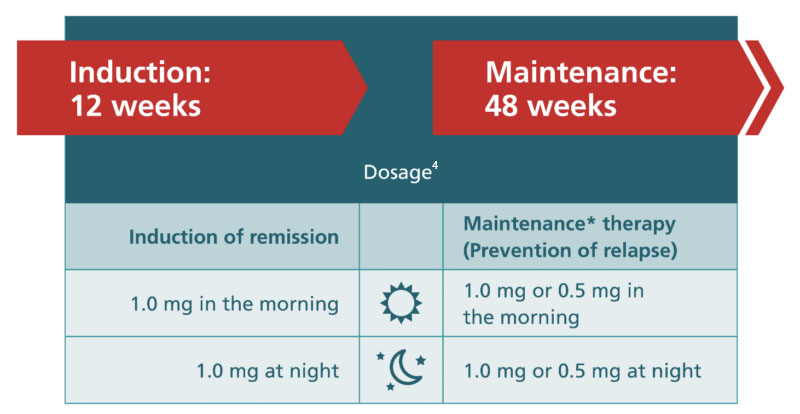
*The maintenance dose of 1.0 mg Jorveza® twice daily is recommended for patients with a long-standing disease history and/or high extent of oesophageal inflammation in their acute disease state.4
Jorveza
® is the
only TGA-approved medication approved for induction and maintenance therapy of EoE in adults. It is available in two strengths: 1 mg budesonide orodispersible tablet and a 0.5 mg budesonide orodispersible tablet.
PLEASE REVIEW PRODUCT INFORMATION BEFORE PRESCRIBING.
COPIES OF THE PRODUCT INFORMATION FOR JORVEZA® CAN BE REQUESTED BY CALLING DR FALK PHARMA AUSTRALIA PTY LTD ON 1800 DRFALK (1800 373 233), OR AT THERAPEUTIC GOODS ADMINISTRATION.
|
PBS Information: Authority required for the treatment of EoE.
Refer to the PBS Schedule for full authority information.
|
3. References
- Lucendo AJ, Miehlke S, Schlag C et al. Efficacy of budesonide orodispersible tablets as induction therapy for eosinophilic oesophagitis in a randomized placebo-controlled trial. Gastroenterology 2019; 157:74–86.
- Helou EF, Simonson J, Arora AS. 3-yr-follow-up of topical corticosteroid treatment for eosinophilic oesophagitis in adults. Am J Gastroenterol. 2008;103(9):2194–9.
- Straumann A, Lucendo AJ, Miehlke S, et al. budesonide orodispersible tablets maintain remission in a randomized, placebo-controlled trial of patients with eosinophilic oesophagitis. Gastroenterology 2020;159(5):1672–1685.e5.
- Jorveza® Product Information.