Proton-pump inhibitors (PPIs) are not approved for the treatment of EoE. Treatment with PPIs leads to clinical and histological remission some adult and paediatric patients.1 The effects of PPIs are likely due to acid-independent inhibition of eotaxin-3, a key cytokine in the pathogenesis of EoE.2 A meta-analysis of 33 studies on the effects of PPIs in the treatment of EoE patients revealed histological remission in 50.5% of subjects and symptom improvement in 60.8%. A higher response tended to be seen with higher PPI doses (standard dose 2x versus 1x daily) and in patients with pathologic pH metrics. However, the authors of the meta-analysis specifically noted the poor quality of the evidence (e.g., no placebo-controlled studies), heterogeneity and significant publication bias.3 A recent prospective study reported clinical and histological remission in only 33% of patients using high-dose PPI therapy with 2 x 40 mg omeprazole/day for 8 weeks. Following the step-down phase to 20 mg omeprazole/day, the remission rate dropped to 12% (see fig. 2).4 There are still no placebo-controlled studies on the use of PPI to treat EoE, rendering it impossible to quantify their general benefit and to formulate a clear recommendation on the length of treatment. Moreover, PPI are currently not approved for the treatment of EoE.
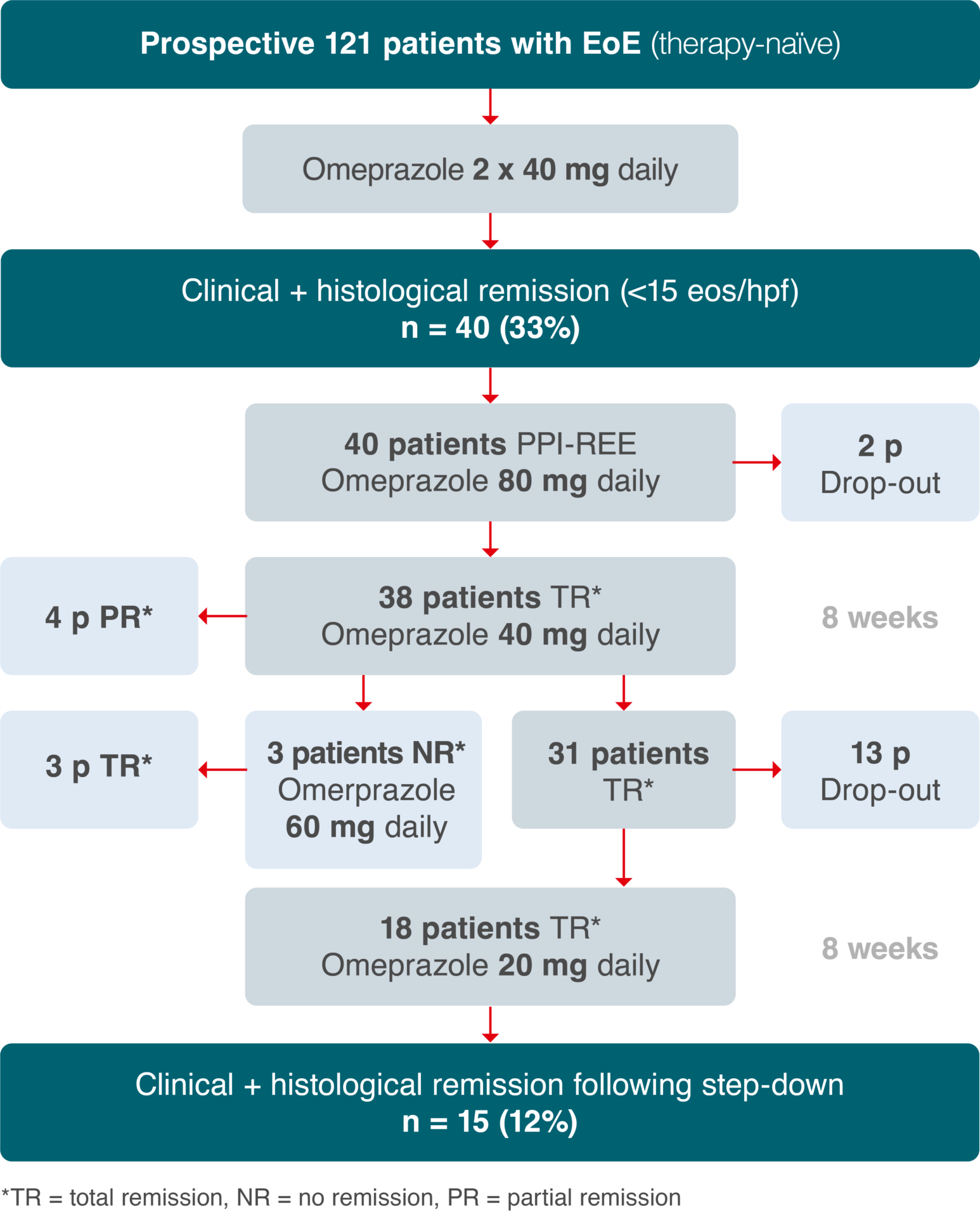
Adapted from Gómez-Torrijos et al., 2016.4